
Pressure units
Various units are used for pressure measurements. Despite the international SI system, specifications such as technical atmosphere (at), physical atmosphere (atm) or psi (pounds per square inch) can still be found in engineering. This page should help you to systematize your knowledge about pressure units.
Table of contents
What is the unit Pascal (Pa)?
Pascal (Pa) is the SI unit for pressure and is used in the definition of atmospheric pressure. If a surface of 1 m2 is loaded with 100 g of weight force (F = 0.1 g ×10 m/s2 = 1 N), this results in a pressure of 1 Pa.*
1 Pa = 1 N/m2
In the remainder of this article, we will also use the abbreviations MPa and hPa, which stand for megapascal (106 Pa) and hectopascal (102 Pa = 100 Pa), respectively.
When to use bar?
The usual unit for higher pressures in Europe is the bar (bar), since Pascal (Pa) is too small a unit of measurement for most technical applications. Below you will find a conversion from 1 bar to the corresponding pressure value in the unit Pa.
1 bar = 105 N/m2 = 100 000 Pa
1 bar corresponds approximately to the pressure exerted by a mass of 1 kg on an area of 1 cm2 or 10 t on 1 m2.
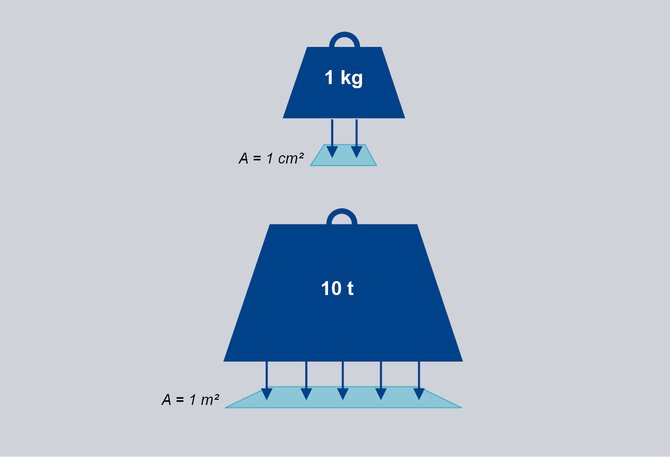
A weight with the mass of 1 kg
loads on an area of 1 cm2.
The resulting pressure is 1 bar.*
A weight with the mass of 10 t
loads on an area of 1 m2.
The resulting pressure is 1 bar.*
*Note: The exact value of the acceleration due to gravity g = 9.81 m/s² was not used here, but a rounded value of 10 m/s².
| convert bar | |
|---|---|
| Value | |
| 1 Pa | 0,01 |
| 1 hPa | 1 |
| 1 psi | 68,97 |
| 1 mWs | 98,07 |
| 1 mmHg | 1,333 |
| 1 Torr | 1,333 |
| 1 at | 980,07 |
| 1 atm | 1013,3 |
What other units are there?
The pressure units at and atm were replaced by the unit bar in 1978 and have since then no longer been among the legal German units of pressure measurement. However, their history is interesting and shows the connection with other, partly still common pressure units:
Technical atmosphere
The technical atmosphere at was based on the hydrostatic pressure exerted by a ten-meter-high water column as a reference. With a density of ρ = 1 kg/dm3 and the acceleration due to gravity of g = 9.80665 m/s2 , the following applies:
1 at = 0,980665 bar = 10 mWs
The technical atmosphere at is usually followed by an index character, depending on whether it is an absolute pressure (ata), a negative pressure (atu) or an overpressure (atü), such as prevails in tire pressure testers at service stations.
Physical atmosphere
The physical atmosphere reference atm is the average atmospheric pressure prevailing at sea level caused by the weight force of the Earth's atmosphere.
Pressure atm:
1 atm = 1.01325 bar = 760 Torr = 760 mmHg
Millimeter of the mercury column (mmHg)
The unit of measurement mmHg is identical to the unit of pressure Torr and denotes the pressure exerted by a column of mercury 1 millimeter high with a density of 13.5951 g/cm3 and an acceleration due to gravity of 9.80665 m/s2.
Unit of pressure mmHg:
1 mm Hg = 133,322387415 Pa
The millimeter mercury column is not one of the SI-compliant pressure units, but is a legal unit used in medicine to diagnose blood pressure in the EU states and Switzerland.
Pound per square inch
The unit commonly used in the USA is pounds per square inch (psi). However, the relevant information in product specifications or data sheets is usually given in psi (pounds per square inch).
Pressure unit psi:
1 psi = 1 ibf/in2, whereby:
ibf - a unit of force (pound-force), defined as the force that attracts a body of 1 pound, assuming that the acceleration due to gravity has a constant value equal to g = 9.80665 m/s2,
1 psi = 6.894,75729 Pa = 6.894 kPa
Convert pressure units
The following information allows you to easily convert between the most common pressure units:
-
1 bar (bar) = 100 000 Pa = 0.1 MPa = 1,02 at = 0,987 atm = 750 Tr = 705,062 mmHg = 14,50377 psi
- 1 megapascal (MPa) = 1000 000 Pa = 10 bar = 10,2 at = 9,87 atm = 7500,637 Tr = 7 500,615 mmHg = 145,038 psi
- 1 pounds per square inch (psi) = 6894,76 Pa = 0,07 bar = 0,07 at = 0,07 atm = 51,71 Tr = 51,71 mmHg
- 1 meter water column (mH2O) = 9806,65 Pa = 0,10 bar = 0,10 at = 0,10 atm = 73,56 Tr = 73,56 mmHg = 1,42 psi
- 1 millimeter mercury (mmHg) = 133,322 Pa = 0,00133 bar = 0,00136 at = 0,00132 atm = 1,000000142 Tr = 0,0193 psi
- 1 Tor (Tr) = 133,322 Pa = 0,00133 bar = 0,00136 at = 0,00132 atm = 0,999999857 mmHg = 0,0193 psi
- 1 technical atmosphere (at) = 98066,50 Pa = 0,981 bar = 0,968 atm = 735,561 Tr = 735,561 mmHg = 14,223 psi
- 1 physical atmosphere (atm) = 101325 Pa = 1,01325 bar = 1,0332 at = 760,002 Tr = 7 760,002 mmHg = 14,696 psi
Pressure units in everyday life
An example of the use of pressure units can be found in the specifications of a pressure gauge, such as the JUMO TAROS S47 P. In the version with a measuring range of 0 to 6 bar relative pressure, the burst pressure is 60 bar. This means that irreparable damage to the instrument will occur at a pressure of 60 bar = 6MPa = 61.183 at = 59.215 atm = 870.226 psi = 37503.18 mmHg.
It is also worth mentioning a pressure measuring device such as a differential pressure transmitter, e.g. a pressure sensor for non-aggressive gases. The input pressure ranges from 0 to 5 mbar. This means that the maximum differential pressure that the sensor can detect is 5 bar = 10-3 Pa = 1 hPa = 0.0051 at = 0.00493 atm = 0.0725 psi.
- ${title}${badge}


